A well-organised Blood Transfusion Service (BTS) is a vital component of any health care delivery system. An integrated strategy for Blood Safety is required for elimination of transfusion-transmitted infections and for provision of safe and adequate blood transfusion services to the people. The main component of an integrated strategy includes collection of blood only from voluntary, non remunerated blood donors, screening for all transfusion transmitted infections and reduction of unnecessary transfusion.
The Blood Transfusion Service in the country is highly decentralized and lacks many vital resources like manpower, adequate infrastructure and financial base. The main issue, which plagues blood banking system in the country, is fragmented management. The standards vary from State to State, cities to cities and centre to centre in the same city. In spite of hospital-based system, many large hospitals and nursing homes do not have their own blood banks and this has led to proliferation of stand-alone private blood banks. The blood component production/availability and utilization is extremely limited. There is shortage of trained health-care professionals in the field of transfusion medicine.
For quality, safety and efficacy of blood and blood products, well-equipped blood centres with adequate infrastructure and trained manpower is an essential requirement. For effective clinical use of blood, it is necessary to train clinical staff. To attain maximum safety, the requirements of good manufacturing practices and implementation of quality system moving towards total quality management, have posed a challenge to the organization and management of blood transfusion service.
Thus, a need for modification and change in the blood transfusion service has necessitated formulation of a National Blood Policy and development of a National Blood Programme, which will also ensure implementation of the directives of Supreme Court of India – 1996.
The transfusion services can be defined as “a medical facility, designed, equipped and staffed, to procure draw, process, and store and distribute human blood and its derivatives”. (American Association of Blood Banks).
An early development leading to the establishment of blood banks dates back to 1915, when Richard Lewison of Mount Sinai Hospital, New York initiated the use of sodium citrate as an anticoagulant. This discovery transformed the blood transfusion procedure from direct (vein-to-vein) to indirect. The introduction of a citrate-glucose solution by Francis Peyton Rous and JR Turner 2 years later permitted storage of blood in containers for several days, thus opening the way for the first “blood depot” established in Britain during World War I. Oswald Hope Robertson, a medical researcher and U.S. Army officer established the depots. Fantus originated the term “blood bank.” Within a few years, hospital and community blood banks were established across the United States. Willem Johan Kolff organized the first blood bank in Europe (in 1940).
An important breakthrough came in 1939-40 when Karl Landsteiner, Alex Wiener, Philip Levine, and R.E. Stetson discovered the Rh blood group system, which was found to be the cause of the majority of transfusion reactions up to that time. Three years later, the introduction by J.F. Loutit and Patrick L. Mollison of acid-citrate-dextrose (ACD) solution, which reduces the volume of anticoagulant, permitted transfusions of greater volumes of blood and allowed longer term storage. An anticoagulant preservative, CPDA-1 was introduced in 1979. It decreased wastage from expiration and facilitated resource sharing among blood banks. Newer solutions contain adenine and extend the shelf life of red cells to 49 days.
Functions of Blood Banks
The main functions of the blood bank are:
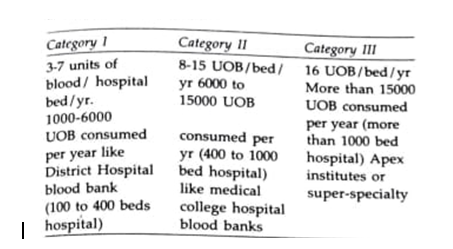
Objectives Of the Policy:
To achieve the above aim, the following objectives are drawn:
The aim of blood transfusion services is to supply good quality of blood and components to the patients and avoid any risk to the donors as well as recipients. Hence it is extremely essential to institute strict quality control measures. The approach to quality management of Blood Bank services is through control of all three aspects of quality, i.e. the Structure, Process and Outcome.
To the patient a good quality outcome means a service that will not let a patient die for want of blood. It means availability of blood:
A high quality of outcome as desired by the patient, however, depends on the quality of Structure (facilities available) and the Process and, to a large extent, also on the availability of voluntary donors.
Ideally should be located on the ground floor and have direct access from main entrance. Signage system should be in place for easy visibility. Blood bank should be located close to the emergency department of the hospital, operation theatre complex, in any case in close proximity of the hospital’s clinical service departments.
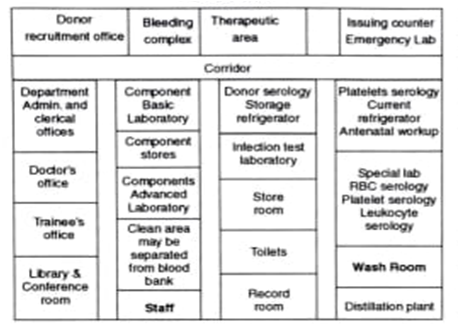
The physical facilities of a blood bank can be grouped under the following areas:
1. Donor recruitment office: Donor recruitment is the most vital activity for any blood bank. Recruiting voluntary donors requires spread of public awareness through mass media or community approach. Keeping in mind the donor recruitment office should have the following facilities:
a. Donor organizer’s space.
b. Space for clerical space.
c. Space for type writer/ printer/fax machine/ photocopier machine.
d. Telephone with intercom facility.
e. Computer for database management for recording and retrieval of data.
f. Facility for printing and publicity material.
2. Bleeding complex: It should be close to the front entrance, to ensure easy visibility, it will consist of the following rooms:
a. Reception room
b. Examination room
c. Bleeding room and apheresis room
d. Rest room
e. Kitchen/pantry
f. Day-care room/treatment room.
3. Therapeutic area: The therapeutic area will consist of the facilities required for blood transfusion of patients in case of those who require frequent transfusion of blood or blood components like patients of Thalassemia or haemophilia.
4. Laboratory: There should be minimum movement of the persons in the corridor of the laboratory and limited access of the outsiders. The processing typing and cross matching of blood is performed in this area. The laboratories of the blood banks are of following types:8
a. Laboratory for processing donor’s blood
b. Blood component laboratory will have to prepare for:
a. Basic work:
b. Advance work:
c. Donors serological laboratory:
d. Infectious diseases laboratory: The laboratory will have provision for testing of:
5. Laboratory for processing patient’s blood
6. Issue counter/room for issue of blood: It should be located at the entrance of the blood bank so that patient’s sample can be received and blood and blood components issued without disturbing the operation of the laboratories.
7. Issue counter/room for issue of blood: It should be located at the entrance of the blood bank so that patient’s sample can be received and blood and blood components issued without disturbing the operation of the laboratories.
8. Patient’s Serological laboratory: The laboratory will do following functions:
a. Emergency function
b. Non emergency:
i. ABO & Rh grouping
ii. Antibody screening
iii. Identification of antibodies in positive screening
iv. Cross matching of blood
v. Antenatal work up.
9. laboratory: Will do platelet and granulocytes serology and HLA work.
a. Administrative area: This area will consist of the following facilities:
b. Teaching and training facilities: If the blood bank is a part of teaching/training institution it will participate in teaching and training program and in some of the hospitals the blood bank is a part of department of transfusion medicine. It will have facilities of:
c. Other physical facilities: In addition to the various facilities mentioned above following things need extra attention as far as blood bank is concerned:
Blood bank equipment’s can be grouped into two:
a.Refrigerator: For maintaining temperature to 1-4° with audio-visual alarm, temperature display, temperature recording, 24 hour power supply number will depend upon the category of blood bank minimum number of refrigerators should be 3
i.Untested blood-1
ii.Tested blood-1
iii.Tested and cross matched blood-1
a. Dielectric sealer-2
b. Vortex mixer
c. Analytical balance
d. Plasma separation stand
e. Magnetic stirrer
f. Laminar air flow
g. Micro plate agitator
a. Blood Bank refrigerator
b. Freezer 70°C with alarm
c. Freezer 20-40°C with alarm
d. Dielectric tube sealer
e. Plasma separation stand
f. Tube stripper, cutter, aluminium agitator
g. Platelet agitator
h. Cryoprecipitate thawing bath
i. 2 kg weighing scale for blood components with sensitivity of 100 mg.
j. 1 kg. Balance with 5 mg. increment for weighing plasma bags
k. Laminar air flow
l. Computer, printer, UPS, and stand by gene rator
Screening of blood equipment’s: ELISHA system with washer, incubator and reader kits for HIV/HBV/ HCV/VDRL tests. Apheresis system when procedure is carried out.
Every blood bank should develop and maintain the written policy of the blood transfusion services/ blood bank of the hospital and procedures being followed in the form of Standard Operating Procedures (SOP). The SOP is the step-by-step approach; it is a form of algorithm. What is to be done and under which circumstances by whom and how? All these questions are answered in a very systematic manner. The policy is nothing but the broad guidelines issued by the top management for day-to-day decision making by the lower-level functionaries. Some important aspects regarding Donor Selection, proceedings with bleeding of donor and processing the donor’s blood are being discussed. The national AIDS Control Organization (NACO), under The Government of India, Ministry of Health and Family Welfare, New Delhi has designed the various procedures and step by step approach while dealing with the transfusion services of the hospital. The students are advised to go through the details of the document to understand the minute procedural details of the blood transfusion services, in the document prepared by NACO, “Standards for blood bank and Blood Transfusion Services”. It is beyond the scope of this book to give all the details; however, the relevant portions are being summarized here for the benefit of the readers.
In order to protect the donor as well as the recipient, each blood donor must be screened prior to each blood donation by medical history and appropriate physical examination on the day of donation. Whenever a decision is made regarding acceptance or rejection of a blood donor, these two things should be kept in mind, i.e.
The donor should have:
Under what circumstances, there can be refusal to take donation of the blood; it can be a permanent deferral or a temporary deferral, depending upon the situation. These are being discussed below:
a. Full name
b. Address
c. Contact details
d. Telephone number
e. Age, sex, and race.
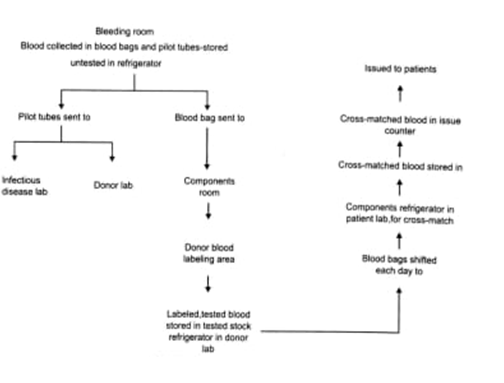
2. Specimens must be labelled at the bedside; with the full name of the patient, date, identification number, and the initials of the person drawing blood. The particulars must match with those given on the requisition from.
3. If the blood sample has to be drawn from the intravenous (I/V) tubing, it should be flushed with saline and the first 5 ml of blood discarded.
4. When additional transfusions are requested, a new specimen should be obtained at each 48-hour interval to identify an incompatibility from an antibody developed by an anamnestic response.
5. Haemolysed blood samples should be avoided.
6. It is good practice to wash red blood cells routinely before testing.
7. ABO and Rh typing should be done on every recipient before blood is issued.
8. Antibody screening is very essential.
9. Cross matching should be done on every unit of blood to be transfused to the patient.
Only an authorized hospital employee (Doctor, Nurse or Nursing orderly) should receive the blood on behalf of the patient. Person receiving blood and technician issuing the blood, should sign in the issue register. Blood should be transfused without delay. All empty bags should be returned to the blood bank within 24 hours of use.
The provision of safe and adequate blood is the responsibility of government. The formation of a nationally organized and managed blood program should be an integral part of each country’s national health care policy and health care infrastructure. The blood transfusion service (BTS) should be established in accordance with an agreed: National blood Policy and plan within a legislative framework. It should be responsible for establishing and maintaining a national quality system, including the development of guidelines and standards, staff training, a data/ information management system and a system for monitoring and evaluation of all the blood transfusion activities.
The Blood Bank or Blood Transfusion Service should have its own constitution, which defines the responsibility and authority of the management.
10 .All containers and anticoagulants used for storage, preservation of blood and blood components and required reagents used for testing of blood samples should meet the standards of Drugs and Cosmetics Act and Rules and Bureau of Indian Standards (BIS).
The blood banks and transfusion services should aim to accept blood from only voluntary non remunerated safe blood donors and to do away with the high risk donors and blood sellers. They should gradually phase out replacement donors. (Blood sellers have been banned as per Supreme Court directive).
The blood banks should establish and maintain a quality assurance system based on any current international standard that includes the following essentials (Fig. 38.3):
Blood donation camps should be organised to augment blood stocks. Donor organiser/medical social worker of the blood bank should contact offices, institutions, industries, social and religious organisations, colleges and schools to collect need based volume of blood from targeted group of donors located at a particular venue at regular intervals. Adequate publicity and IEC material should be made available to the organisations. The number of blood units collected should commensurate with the actual requirement of blood units rather than by social or emotional pressures. The donation site should be inspected prior to the day of blood collection to ensure availability of all facilities as prescribed by Drugs and Cosmetics Rules. The outdoor camp should be organised an environment which is conducive and comfortable. The area should be cleaned before and after the blood collection. Blood bank should maintain quality at each step from donor recruitment, selection and collection to the final product. The method of blood collection and management of donors should be the same as at fixed sites. Blood and its components could contain infectious agents and hence these should be handled with precautions. The large camps organised on a day should be well planned as per criteria laid down by the Drugs & Cosmetics Act. All quality measures and pre donation counselling procedures should not be compromised.
Apheresis is a procedure carried out to harvest a particular component and returning the rest of the blood to the donor, by an automated machine. This procedure should be carried out only in a blood bank licensed for this purpose.
The guidelines relate only to apheresis of healthy voluntary donors and not to any therapeutic procedure. A medical officer trained in apheresis technique should be responsible for the procedure. There should be provision for emergency medical care, in the event of any adverse reaction to the donor. The staff working on the machine should be trained in apheresis procedure and should work directly under the supervision of the medical officer. The donor should be asked to sign a consent form in the language, which he understands after being explained the procedure and the risks involved.
It is a procedure to harvest plasma from the whole blood and returning the cellular components to the donor. Plasma is harvested by automated machine.
In an occasional Plasmapheresis in which donors undergo the process once every 12 weeks the standards for whole blood donation should apply.
In a ‘serial’ Plasmapheresis in which plasma is donated more frequently than once every 12 weeks, the donor should be tested before every apheresis procedure – Hemoglobin and/or hematocrit should be > 12 g/dl and/or Hct 36% – Total serum protein should not be below 6.0 gm/dl. In serial plasmapheresis program with return of the cellular components a minimum interval should be of 48 hours between two procedures and not more than two procedures in a week should be allowed. If a participant of such program donates a unit of blood or if it is not possible to return red cells, the donor should not undergo platelet/Plasmapheresis for 12 weeks.
Records of donor’s periodic examination, laboratory tests, consent of donor/patient, date of last apheresis procedure, certificate of the attending physician, procedure, volume of product separated, drugs used, adverse reaction if any and their treatment should be maintained.
Volume of plasma obtained excluding anticoagulants from a donor weighing at least 55 kg, should not exceed 500 ml with serum protein within normal limit during one procedure or not more than 1000 ml per month with a maximum of 12 L/year. Extra corporeal blood volume should not exceed 15% of donor’s estimated blood volume.
Cytapheresis is the procedure for separation of individual cellular component of blood. It can be achieved by the cell separator machine. Plateletpheresis is the harvesting of platelets from whole blood using continuous or intermittent flow cell separator. Leukapheresis is the harvesting of granulocytes from whole blood using continuous or intermittent cell separator. Peripheral blood stem cells are harvested using continuous or intermittent cell separator. Attempt should be made for harvesting minimum of 2×106 CD34 cells and/or minimum of 2×108 MNCS/Kg of the recipient,
Donors who undergo Cytapheresis, not more than once every 4 weeks should be treated as ordinary blood donors with regards to laboratory studies. Donors, who undergo serial cytapheresis, more than once every 12 weeks, should be tested as under. Hemoglobin and/or haematocrit should be > 12 g/dl and or Hct of 36%. Total serum protein should not be below 6.0 gm/dl. It should be tested before the 3rd collection if done within 4 weeks. Platelet count should be determined before plateletpheresis and should not be below 150,000/dl. Total and differential white cell count should be normal. Persons who have ingested aspirin or similar anti platelet drugs in the last 72 hours should not be suitable for Plateletpheresis. Donors with personal and family history of bleeding tendency should not be suitable for Plateletpheresis. Before leukapheresis total white blood cells counts should be 4000/dl with normal differential count. In serial pheresis a minimum interval should be of 48 hours and not more than two procedures in a week should be allowed. A participant of such a program donates a unit of blood or if it has not been possible to re-infuse the red cells during a pheresis procedure should not be accepted for cytapheresis for 12 weeks.
Extracorporeal blood volume should not exceed 15% of the donor’s estimated blood volume. Interval between two Cytapheresis should be 48 hours and not more than twice a week. The donors should be tested appropriately to detect a developing cytopenia. Red blood cell loss incidental to the procedure should be no more than 25 ml per week. Donors may receive drugs to facilitate leukapheresis. Such drugs should not be used for donors whose medical history is suggestive of some disease. Donors should be observed closely during cytapheresis as regards the untoward reactions like headache, fainting attack, tachycardia, twitching, dyspnoea etc. Written standard criteria used to determine donor suitability, procedure of hemapheresis, precautions to ensure reinfusion of donor’s own red cells, and time frame should be maintained.
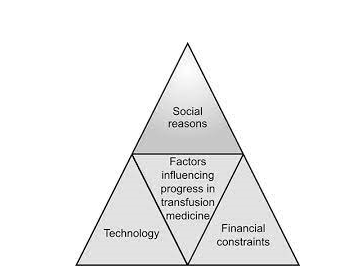
The factors, which influence the progress in transfusion medicine, can be viewed broadly under three major groups, which are the issues of great managerial importance.
During these years two fundamental changes in donor recruitment have taken place: a shift from individual responsibility to community responsibility for blood replacement and from paid donors to voluntary donors. As the organizing involves three basic functions that is:
Organization of a blood transfusion service/ blood bank is possible only when there is an assured supply of voluntary blood round the year. The most important managerial issue of a blood bank is the donor recruitment and bleeding donors. Success in donor recruitment and maintaining donor records requires an effective media communication by the health care professionals; the students are advised to consult the chapter on effective media communication in the same book. Effective publicity and educational campaign by means of mass media, i.e. e television, publicity campaign, letters and telephonic calls to schools, colleges, service oriented organizations, voluntary organizations, etc. This work e requires trained personnel headed by a donor organizer including, social workers, nurse, clerical e staff, good communication system and transport s facilities. The various activities performed by the department of transfusion medicine or the blood bank of a hospital, the following chart depicts all these areas diagrammatically.
Based on present usage pattern, if about 1% of the population donates 1 unit of blood every year the nation’s blood needs would be met. To mobilize the 1% population is a challenging job and coordination of great degree is required to accomplish this objective.
All the principles related to the inventory control are s equally applicable in the transfusion medicine also.The basic objective is to select right kind of donors, with right methods and techniques, at right time for right people and transfused rightly. The management aspect starts from the step of demand estimation, to procurement, stocking and distribution system of blood. Ideally no blood or blood components should be allowed to outdate. Although it is impractical or even impossible in small hospitals, but every effort should be made to reduce such waste. As per the available data the wastage due to outdating of blood is to the tune of about 10 %, which is a significant figure, in the light of such a scarce commodity.
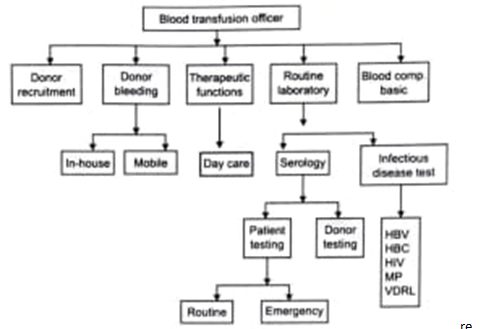
a. Training of technical personnel
b. Post graduate course
c. Drug inspectors training
4. Institution for conducting research on various areas of transfusion medicine.
5. Banning unlicensed blood banks within 1 year.
6. Banning professional donors within 2 years.
7. Income tax exemptions for donations to councils viii. NACO/coordinating the development of developing a national blood transfusion network in India.
8. Hindustan Latex Ltd. Engaged to assist NACO in preparation of a comprehensive plan for setting up of national and state councils.
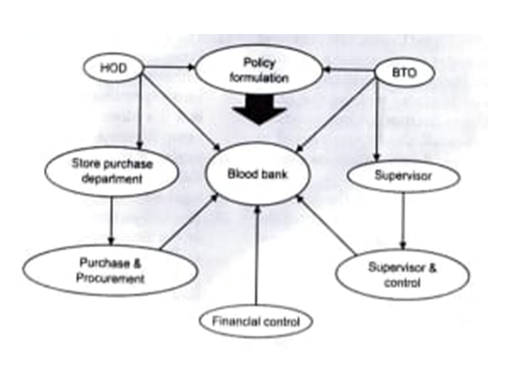
The following diagram illustrates the coordination and control mechanisms in a blood transfusion unit
Cohn and associates during second World War were the pioneers in the fractionation of plasma into its components.
Various components prepared in modern blood banks are:
Blood transfusion reactions are not very uncommon, these ranging from minor reactions like rigors; to severe reactions of fatal in nature. All measures should be undertaken to prevent such reactions by the people working in the blood banks and those involved in the transfusion process. The occurrence of a transfusion reaction should be immediately reported to the blood bank. The reporting authority should send:
a. The laboratory and blood bank records.
b. The blood bank should take urgent steps to establish the cause of the transfusion reaction. The records of transfusion reactions should be maintained.
Exposure of employees to blood and other potentially infectious materials (OPIM) due to ineffective Exposure Control Plan (ECP).
a. Documenting an annual review and update of the written plan that reflects changes in technology for safer medical devices. Employers must also document consideration and implementation of the safer medical devices annually.
b. Employer must get input for the devices. From those responsible for direct patient care. This input must also be documented.
Regulations for governing the blood transfusion services are very strict. Unless all the requisite infrastructure and processes (policies, procedures, protocols) are in place and are of approved standards, the license to operate is denied. However, still there are many unlicensed blood banks operating in the country with dubious quality services.
It is very important to realize the uniqueness of blood as a therapeutic agent. Unlike the drugs, it is a vital and very sensitive item with a short shelf life, is easily spoilt but is not replaceable from the market. It can cause serious damage, even death, if the quality of services and their reliability is compromised. It is therefore of utmost importance to ensure that there is no compromise on quality in any aspect and the blood supplied for transfusion is safe from all the hazards so that it can fulfil its intended purpose- restoring life to the patient.