Laboratory services in medicine can be broadly divided into two categories:
The histopathology section prepares and examine tissues to provide data on cause and progress of disease; makes microscopic examinations of tissue pathology; engages in research to develop new histopathological methods and new stains to produce greater clarity during examination of special tissue structures or chemical components; performs autopsies and interprets gross and microscopic autopsy findings in conference with medical staff and technologists for future diagnoses and treatment of patients.
The biochemistry section performs chemical tests of body fluids and exudates to provide information for diagnosis and treatment of disease; investigates chemical processes involved in functioning and malfunctioning of the human body; and studies effects of chemical compounds upon physiological and biochemical functions of the body to provide information on optimum methods of treating pathological conditions.
The haematology section analyses and test blood specimens and interprets test results to provide a basis d for treatment of diseases; and engages in research related to haematological methods and diagnosis. The blood bank in a hospital having these special units, are usually a part of Hematology. The bank provides for storage and preservation of blood plasma. Blood may be procured either directly from donors or from other blood banks. When obtained from donors, this unit extracts blood and makes necessary laboratory tests to certify its fitness for transfusion.
The microbiology section cultivates, classifies and identifies micro-organisms found in body fluids, exudates, skin scrapings, or autopsy and surgical specimens to provide data on cause, cure, and prevention of disease; engages in research to develop new or improved methods for discovering and identifying pathogenic organisms; and investigates biology, distribution and mode of transmission of bacteria and nature and efficiency of chemotherapeutic treatment. Bacteriology also comes under microbiology.
The serology section prepares serums used to treat and diagnose infectious diseases and immunize against these diseases, and identifies diseases based on characteristic reactions of various serums; investigates safety of new commercial antibiotic products and accuracy of therapeutic claims; direct immunology tests and injections; investigates problems of allergy; and conducts tests to determine therapeutic and toxic dosages and most effective methods of administering serums, vaccines, antibiotics, antitoxins, antigens and related drugs. Air-conditioning of this laboratory is necessary.
Virology is the study of various viruses, which produce pathogenic conditions in the human body. Tests like Western Blot to confirm AIDS infection, study of hepatitis, rabies virus infections, etc. are done in virology laboratory. Air-conditioning is required.
The cytology section examines human cells to detect evidence of cancer in its early stages and other diseased conditions; engages in research to develop new cytological methods and new stains to produce greater clarity during examination of cell structures. The clinical laboratories may be responsible for a number of other functions, such as bawl metabolism tests; activities of clinical photo chromic laboratory, medical illustration unit and mortuary and care and treatment of animals used in research Teaching programs for student nurses, interns, residents, and medical technologists and other laboratory personnel may also be a function of the department.
The pathology laboratory is a very big set up in the large hospital especially if, it is associated with a teaching institution. The components of the laboratory can be enumerated as follows:
1. Morphological pathology:
a. Morbid anatomy (autopsy)
b. Histopathology
c. Exfoliate cytology.
2. Clinical pathology:
a. Determination of blood sugar
b. Lipids, proteins, electrolytes
c. Examination of urine and other body fluids
d. Determination of hormones and enzymes.
3. Microbiology:
a. Bacteriology
b. Parasitology
c. Mycology
d. Virology
4. Haematology:
a. Study of blood
b. Study of bone marrow
c. Study of reticuloendothelial system
d. Laboratory procedures associated with blood transfusion.
5. In some places additional fields are being developed like:
a. Medical biophysics
b. Clinical physiology
Procedures include preparing standard solutions, cross-matching blood donors and recipients, and preservation and storage of specimens. Respective procedures include test purpose, equipment and materials, method, specimen’s collection (time and container type), specimen size and retention period, patients’ preparation (food and drink restrictions), location for obtaining specimens (in laboratory or at bedside), and normal range for test results. The routine procedures are as follows:
Adequate fresh air and removal of noxious and toxic fumes is essential for workers’ health. Lighting should be adequate for matching of colours and accurate reading of fine gradations. Danger can occur from careless handling of infected specimens and cuts from equipment. Errors in test results can occur if patients do not report, for example, their use of proprietary pain relievers.
The factors which determine the type, size and organization of a laboratory are as:
The WHO has recommended following criteria for organizational structure of the laboratories:
The WHO has suggested grouping of laboratories in three categories, i.e.:
Rules Include
Standards such as adequate equipment, materials, space for technicians derive from regulatory agency constraints. Reagents and stains should be of recognized standard. The standard times for performing tests, without hurrying which can cause errors and necessitate re-testing, individually or in batches, manually or electronically, need determining. A standard format for procedures conserves time and other resources.
All workers are on duty five days a week during the day with professional workers ‘on-call’ to provide a twenty-four-hour service everyday. Immediate examination of surgical department tissues may be needed. Pre-admission tests may be performed during evenings. All technicians in specialist divisions should be able to perform routine tests in other divisions.
Sufficient staffing is provided in early mornings to collect antecibum (AC) and corresponding post cibum (PC) blood specimen from large number of patients. Split duties are arranged to cover busy and nonbusy hours.
The laboratory’s product is information. The objective of any laboratory information system is to present data in the most orderly, legible, and timely manner. Clinical laboratory information systems are rapidly becoming highly automated.
In modern hospitals, results of various examinations with reference to the identification number and name of the patient fed into the local area network computer system are made accessible to clinicians through terminals provided in clinics and wards. This provides quick transfer of information to speedup treatment procedures.
| Manual System | Automatic System |
| Requisitioning | Requisitioning |
| · Physician orders laboratory test on physician order sheet. | · No change |
| · Nursing personnel prepares requisition. | · Order via hospital information system |
| · Requisition transported to lab | · Activity eliminated |
| Specimen procurement | Specimen procurement |
| · Requisition time stamped when received | · Activity automated |
| · Label tube | · Same (using computer labels) |
| · Stamp or write time of collection | · Function automated, improved quality by forcing user response |
| Specimen preparation | Specimen preparation |
| · Label pour-off tubes & sample cups | · Use printed labels |
| · Log in specimen | · Actively automated |
| · Enter Requests on worksheets | · Actively automated |
| Analysis & Data Handling | Analysis & Data Handling |
| · Perform analysis | · No change |
| · Record results on worksheets | · No change for manual test, automated tests are run online |
| · Record results on log | · Actively automated |
| · Data review by supervisor | · Process made easier due to online delta checks |
| Reporting | Reporting |
| · Separate forms | · Actively eliminated |
| · Sort by nursing station | · Actively automated |
| · File/distribution | · Actively eliminated |
| · Write or tape results on summary sheets | · Actively eliminated |
| · Chart multiple documents | · Process simplified by handling only one document |
| Financial Data | Financial Data |
| · Sort charge tickets | · Actively eliminated |
| · Keypunch charges into hospital information system | · Actively eliminated |
| Management reporting | Management reporting |
| · Compile monthly statistics | · Actively eliminated |
Perfect administration of blood and blood products should be maintained by following procedures very strictly and avoiding administering contaminated or improperly collected blood. The hospital should have a committee with pathologist as a member to establish written procedures for the use of blood and blood derivatives, including identification and compatibility testing of blood, criteria for use and review of all transfusion reactions occurring in the hospital. An alarm system must be instituted to notify personnel of the power failure and abnormal temperature conditions, which may spoil bloodstock. For AIDS test, the hospital can have its own testing facilities with approved equipment and reagents. Positives on ELISA must be confirmed by Western Blot test and authenticated by the pathologist. Only blood in perfect condition should be allowed for either donation or for transfusion.
The main measure of quality control is the number of tests performed. It may be analysed by test type, clinical service, inpatients and outpatients, and treating physician. Other measures include post mortems performed, pints of transfusion blood prepared, medical photographs and illustrations produced, and materials used.
Controlling includes extent of specimens brought to department between scheduled collections, and whether technicians are offering a diagnosis when telephoning test results to wards. Other controlling includes reasons for increase in number of tests performed (for example, increased admissions, research needs, and community increasingly law suit conscious). An increase in number of tests for each patient stay is related to a decreasing average length of patient stay.
Coordination is needed with the surgical department to know what tissue to expect. It is needed with the public relations department for informing community of need for blood donors.
Wrong results may prove fatal and liable for severe consequences. The laboratory must receive special attention to ensure efficiency and accuracy. Tests should be performed according to established procedures on the basis of several factors such as the number and types of tests ordered, the time they are ordered, etc. Some factors relating to quality maintenance:
It is usually associated to a teaching hospital and situated in big cities. This laboratory will consist of almost all the possible units and it works as a referral laboratory. Research work is an important component in such laboratories.
It should be situated centrally for easy access of the clinicians. The requirement of the space is dependent upon the types of facilities available.
The staff of regional laboratories will consist of medical professionals, non-medical professional, technical and non-technical both. The staff strength will again depend upon the type of the facilities and work load.
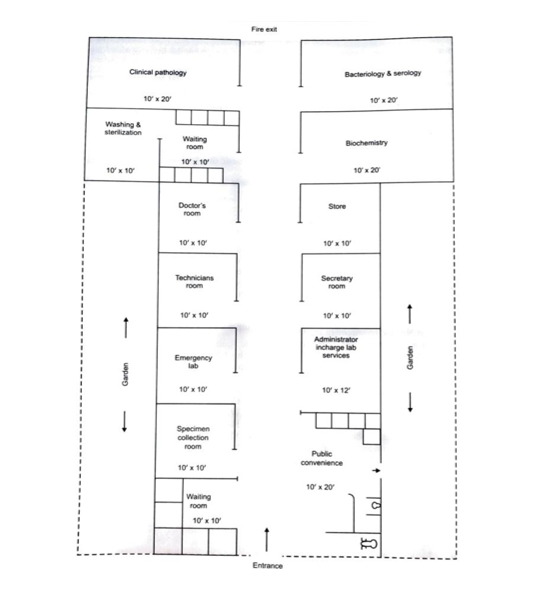
| SI. No. | Name of Facility | No of Room | Size | Area in sq. feet |
| 1 | Administrative area | 1 | 12*10 | 120 sq. feet |
| Toilet facility | 1 | 8*5 | 40 sq. feet | |
| Clerk/ secretary/ record room | 1 | 12*10 | 120 sq. feet | |
| 2 | Laboratory area | 1 | 10*20 | 200 sq. feet |
| Clinical pathology | 1 | 10*20 | 200 sq. feet | |
| Biochemistry, Bacteriology & Serology | 1 | 10*20 | 200 sq. feet | |
| 3 | Service area | 1 | 10*10 | 200 sq. feet |
| Washing & sterilization Storage | 1 | 10*10 | ||
| 4 | Patient area | 1 | 10*10 | |
| Reception area & waiting | 1 | |||
| Specimen collection | 10*10 | 200 sq. feet | ||
The requirement of the staff is calculated on the basis of number of investigations performed. Ideally the staff requirement can be evaluated on the basis of activity sampling and work studies. The formula is used to determine the investigation/day/ technical staff.
Standard time = Total basic time x time allowance
Total basic time = Total average basic time of job element.
Basic time = average observed time x rating
Time allowance = contingency allowance (5 % of total basic time) x Relaxation time (12 % of basic time)
Rating of person at 100% by an average working day functioning technical staff = 6½ hours of duty (36 hours per week). Observed work/day/technical staff when both automation/conventional techniques are shared in mixed type of spectrum of investigation = 60-70 investigation/day.
Basic equipment’s/instruments must be provided in all the laboratories. Following are some of the equipment’s/instruments and reagents of the common use:
The requirement of various types of laboratory equipments depends upon the type and facilities of E laboratory and also work load. Some of the factors are enumerated as follows:
Quality control is a process for assessing the accuracy and reliability of test system. In a clinical laboratory, this is usually accomplished by running chemistry against a specimen with known value (standard, calibrator or control), along with patient’s unknown samples to determine whether the analytical system is functioning within prescribed limits.
Quality control in a clinical laboratory has been defined as the study of errors and the procedures used to recognize and minimize them It is surveillance process in which action of people and performance of equipment and materials are observed in a systematic and periodic way so that it provides a record of consistency of performance and action taken when performance does not conform to standards established in the laboratory. In other words quality control focuses mainly on technical, procedural and process issues.
Quality assurance has been used to represent the techniques available to ensure with a specified degree of confidence that the results reported by the laboratory are correct.
For any clinical laboratory there should be a well written quality control policy, which should include a description of:
Me rule for accepting or rejecting a run should be early stated in the quality control policy. For qualitative tests, the quality control rules are simple. He positive control must be positive and negative Control must be negative for the patient’s result to e valid.
The use of sample obtained from the pool of serum with known values for analysts for the verification of laboratory analysis was introduced in 1970’s and s still the most direct and widely applied quality control technique.
Quality control can be divided into two major types:
In clinical laboratories the use of Mean and Range charts have good performance characteristics.
The mean of a set of observation is defined as sum of all the observations divided by the total number of observations, i.e.
Mean = sum of observations/ no of observations
If x1, x2, x3,……….xN are N observations
The mean’s given as
x̄ = x1 + x2 +x3+……….+xN /N
x̄ = Σxi /N
i= 1, 2, 3, 4………N
It is applied in the clinical laboratory as the Target value for particular analysis of study.
Standard Deviation: It is also called Root Mean Square Deviation It is denoted by Greek letter sigma or simply SD. It is derived by the following formula:
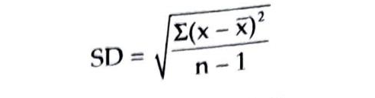
Steps in calculation of
It is the percentage of variance observed in a set of values.
CV (%) = (SD/Mean) x 100
The coefficient of variance (CV) measures the precision of the test (replicates) performed.
Low CV = Increased assay precision
High CV = Reduced assay precision
External quality assessment refers to a system in which laboratory results are scrutinized objectively by an outside agency in order to get a general impression of the standard of laboratory practice and to achieve inter-laboratory comparability. There are two types of programs in which a laboratory can compare their analytical results.
Most well established big branded laboratories participate in various different external quality control program which include:
Accreditation is the process by which an organization is formally recognized and certified by an authoritative body as meeting standards established by experts. It involves a laboratory’s overall quality system and its technical capability. Accreditation agencies review and organizational setup for the quality of its services and certify that the organization meets a set of standards. By asking for accreditation, an organization agrees to be measured against standards set by professionals. Setup for the quality of its services and certify that the organization meets a set of standards. By asking for accreditation, an organization agrees to be measured against standards set by professionals.
Accreditation is a series of good team work done right from the management to the all-laboratory staff to maintain the quality standards at the best expected.
Formal recognition of competence of a laboratory by an accreditation body in accordance with international criteria has many advantages:
For diagnostic setups in India, Government of India. Has authorized National Accreditation Board for Testing and Calibration Laboratories (NABL) as the sole accreditation body for Testing and Calibration laboratories.
NABL is an autonomous body under the Dept. of Science and Technology, Government of India. It provides laboratory accreditation services to laboratories that are performing tests calibrations in accordance with NABL criteria, which is based on internationally accepted standards and guidelines. NABL promotes, coordinates, implements and maintains an accreditation system in the country comparable to relevant national and international standards in order to ensure accuracy, reliability and reproducibility of test results.
This is again a quality control method, in which we lay emphasis on total organization wide change in the concept of quality. It is dependent upon the following parameters:
It is a disciplined, data-driven approach and methodology for eliminating defects (driving towards six standard deviations between the mean and the nearest specification limit) in any process-from manufacturing to transactional and from product to service) The Six Sigma describes quantitatively how a process is performing. To achieve Six Sigma, a process must not produce more than 3.4 defects per million opportunities. A Six Sigma defect is defined as anything outside of customer specifications. Six Sigma has two key methodologies. DMAIC and DMADV. DMAIC is used to improve an existing business process. DMADV is used to create new product designs or process designs in such a way that it results in a more predictable, mature and defect free performance. Sometimes, a DMAIC project may turn into a DFSS project because the process in question requires complete redesign to bring about the desired degree of improvement. When we use statistical methods like standard deviation, six sigma techniques or other statistical tools for quality assurance it is called statistical quality control. The standard deviation is one such tool for statistical Quality Control (SQC).
The autopsy room is related to the laboratory as it functions under the control of the pathologist. When the hospital performs a post-mortem examination. It generally faces difficulty in securing the permission of a deceased person’s next of kin or guardian before the actual post-mortem is performed. It is advisable to obtain a written permission for legal protection. This can be in a standard form. A committee of the medical staff should review all deaths in the hospital. The following facilities are required for autopsy room and mortuary:
Laboratory personnel are more exposed to infections as they handle pathogenic specimens. They should use all safety provisions to avoid cross-infections. They should:
Specimen without Request
Authorized persons should collect specimens after verifying request and clinical data.
Specimen without Identification Data
All specimens should be collected in the clinic itself with identification details in the presence of nursing staff.
Lack of Clinical Data
Written instructions should be given to all clinicians and nursing staff regarding this. Patients Handling Urine and Faeces Specimen. The receiver should confirm all required information before receiving the specimen (ID of the patient, request, to confirm when early morning or midstream urine, etc.).
Improper Specimen Collection
Orient staff repeatedly and supervise closely in the cases of arterial blood/venous blood, minimum quantity of specimen required for various tests.
Punctuality in Collecting Specimen
Give clear instruction to patient in local language in an emphasizing manner. Hemolyzation of blood due to delay in handling specimen. Use specified containers and reagents and store them in specified places.
Damage and Transit loss of Specimen
Ensure proper packing and handling and caution notes.
Transporting Specimen to Higher Centres at Long Distances
Plan duration of transit time, pack in appropriate containers to withstand transit duration and ensure delivery at the right time to the right person with required caution notes.
Skill mix, workstation configuration, workload, and workflow determine the operational characteristics of clinical laboratories. Service levels determine workload and skill mix. Workload and skill mix affect organizational structure, manpower needs, information systems design, physical plant, and equipment needs. All of these operational characteristics provide the basis for determining what goals and objectives the planning process must develop and implement to keep the hospital laboratory a viable, state-of-the-art operation.