a. Planning
b. Formulation
c. co-ordination
d. implementation, conduct or promotion of biomedical research in India.
2. In 1911, the Government of India set up the Indian Research Fund Association (IRFA) with the specific objective of sponsoring and coordinating medical research in the country.
3. After independence, several important changes were made in the organization and the activities of the IRFA
4. It was redesignated the Indian Council of Medical Research (ICMR) in 1949, with expanded scope of functions & responsibility.
5. The ICMR is funded by the Government of India through the Ministry of Health and Family Welfare.
6. The council not only plans & promotes medical research & emerging health problems of the country but also is expected to build strong national group of professional persons in organization of skilled biomedical scientists.
7. Rapid advances in science & technology have brought in new technologies to understand the disease process & find strategies for prevention & cure.
8. The policies of ICMR coincide with national health policies such as
9. Control and management of communicable diseases,
10. fertility control,
11. maternal and child health,
12. control of nutritional disorders,
13. developing alternative strategies for health care delivery,
14. safety limits of environmental and occupational health problems,
15. Non-communicable diseases including cancer, cardiovascular, neurological, ophthalmic and haematological disorders.
ICMR, with its 30 state-of-the-art Institutes/Centres, is amongst research organizations in the field of Bio Medical Sciences.

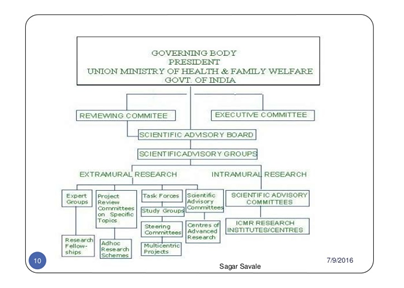
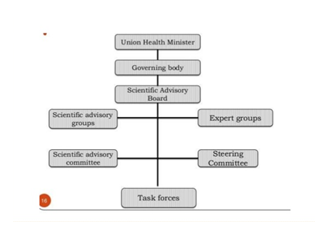
a. Union Health Minister.
b. Scientific Advisory Board.
i. Scientific Advisory Group
ii. Scientific Advisory Committees
iii. Expert Groups.
iv. Task Forces.
v. Steering Committees etc. which evaluate and monitor different research activities of the Council.
a. Intramural research is carried out currently through the Council’s 30 Research Institutes/Centres.
b. These include 19 mission-oriented national institute:
i. National Jalma institute for Leprosy & Other Mycobacterial Diseases (NCJILOMD), Agra.
ii. National Institute of Occupational Health (NIOH), Ahmadabad.
iii. Tuberculosis Research Centre (TRC), Chennai
iv. National Institute of Malaria Research (NIMR), Delhi
v. National Institute for Research in Tuberculosis (NIRT), Chennai
vi. National Institute of Pathology (NIP), Delhi
vii. National Institute of Medical Statistics (NIMS), Delhi.
viii. National Institute for Research in Environmental Health (NIREH), Bhopal
ix. National Centre for Disease Informatics and Research, Bangalore
x. National Institute for Research in Reproductive Health (NIRRH), Mumbai
xi. National Institute of Immunohematology (NIIH), Mumbai
xii. Enterovirus Research Centre (ERC), Mumbai
xiii. Institute of Cytology and Preventive Oncology (ICPO), Noida
xiv. Rajendra Memorial Research Institute of Medical Sciences (RMRIMS), Patna
xv. Vector Control Research Centre (VCRC), Pondicherry
xvi. National Institute of Virology (NIV), Pune
xvii. National AIDS Research Institute (NARI), Pune.
xiii. Regional Medical Research Centres:
Extramural research is promoted by ICMR through Setting up Centres for Advanced Research in selected departments of Medical Colleges, Universities and other non-ICMR Research Institutes.
These statements of General and Specific Principles may be varied, amended, substituted and added from time to time.
Medical and Related Research on human participation must ensure that:
Principles
a. Principle of voluntariness
b. Principle of non-exploitation
c. Principle of social responsibility
d. Principle of ensuring privacy and confidentiality
e. Principle of risk minimization
f. Principle of professional competence
g. Principle of maximization of benefit
h. Principle of institutional arrangements
i. Principle of transparency and accountability
j. Principle of totality of responsibility
k. Principle of environmental protection
Principle of Essentiality: Whereby after due consideration of all alternatives in the light of existing knowledge, the use of human participants is considered to be essential for the proposed research. This should be duly vetted by an ethics committee (EC) independent of the proposed research.
Principle of Voluntariness: Whereby respect for the right of the participant to agree or not to agree to participate in research, or to withdraw from research at any time, is paramount. The informed consent process ensures that participants’ rights are safeguarded.
Principle of Non – Exploitation: Whereby research participants are equitably selected so that the benefits and burdens of the research are distributed fairly and without arbitrariness or discrimination. Sufficient safeguards to protect vulnerable groups should be ensured.
Principle of social responsibility: Whereby the research is planned and conducted so as to avoid creation or deepening of social and historic divisions or in any way disturb social harmony in community relationships.
Principle of ensuring privacy and confidentiality: Whereby to maintain privacy of the potential participant, her/his identity and records are kept confidential and access is limited to only those authorized. However, under certain circumstances (suicidal ideation, homicidal tendency, HIV positive status, when required by court of law etc.) privacy of the information can be breached in consultation with the EC for valid scientific or legal reasons as the right to life of an individual supersedes the right to privacy of the research participant.
Principle of risk maximization: whereby due care is taken by all stakeholders (including but not limited to researchers, ECs, sponsors, regulators) at all stages of the research to ensure that the risks are minimized and appropriate care and compensation is given if any harm occurs.
Principle of professional competence: whereby the research is planned, conducted, evaluated and monitored throughout by persons who are competent and have the appropriate and relevant qualification, experience and/or training.
Principle of maximization of benefit: whereby due care is taken to design and conduct the research in such a way as to directly or indirectly maximize the benefits to the research participants and/or to the society.
Principle of institutional arrangements: Whereby institutions where the research is being conducted, have policies for appropriate research governance and take the responsibility to facilitate research by providing required infrastructure, manpower, funds and training opportunities.
Principle of transparency and accountability: Whereby the research plan and outcomes emanating from the research are brought into the public domain through registries, reports and scientific and other publications while safeguarding the right to privacy of the participants. Stakeholders involved in research should disclose any existing conflict of interest and manage it appropriately. The research should be conducted in a fair, honest, impartial and transparent manner to guarantee accountability. Related records, data and notes should be retained for the required period for possible external scrutiny/ audit.
Principle of totality of responsibility: Whereby all stakeholders involved in research are responsible for their actions. The professional, social and moral responsibilities compliant with ethical guidelines and related regulations are binding on all stakeholders directly or indirectly.
Principle of environmental protection: whereby researchers are accountable for ensuring protection of the environment and resources at all stages of the research, in compliance with existing guidelines and regulations.
The ICMR encourage human resources development in biomedical research through various schemes such as
Grant Schemes
Short Term Research Studentship
2. Fellowships/ Associateships
Research Fellowships/ Associateships
Junior Research Fellowships
The schemes aim at promoting good quality research in medical colleges thought students pursing post graduation courses as well as improve visibility & accessibility of their research work.